Abstract
Background: CAD is a rare autoimmune hemolytic anemia mediated by classical complement pathway activation. Sutimlimab, a first-in-class humanized monoclonal anti-C1s IgG antibody, selectively inhibited the classical pathway, halted hemolysis, and improved hemoglobin (Hb) and quality of life (QOL) in patients with CAD and recent transfusion history (single-arm CARDINAL study [NCT03347396]). CADENZA (NCT03347422) is a 26-week (Part A) randomized, double-blind, placebo (PBO)-controlled Phase 3 study with open-label extension (Part B) to assess sutimlimab in patients with CAD without recent transfusion history.
Aim: To report efficacy and safety results from CADENZA Part A.
Methods: Patients with confirmed CAD diagnosis, baseline (BL) Hb ≤10 g/dL, bilirubin above normal, transfusion independence, and ≥1 CAD symptom were enrolled. Patients were randomized 1:1 to receive sutimlimab (body weight <75 kg, 6.5 g; ≥75 kg, 7.5 g; N=22) or PBO (N=20) on Days 0 and 7, then biweekly infusions. The composite primary endpoint (sutimlimab vs PBO compared using the Cochran-Mantel-Haenszel method) was the proportion of patients with Hb increase ≥1.5 g/dL at the treatment assessment timepoint (TAT; mean of Weeks 23, 25, and 26) and avoidance of transfusion and study-prohibited CAD therapy (Weeks 5-26). Secondary endpoints included markers of hemolysis, Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), and pharmacodynamic (PD) outcomes. Safety was evaluated.
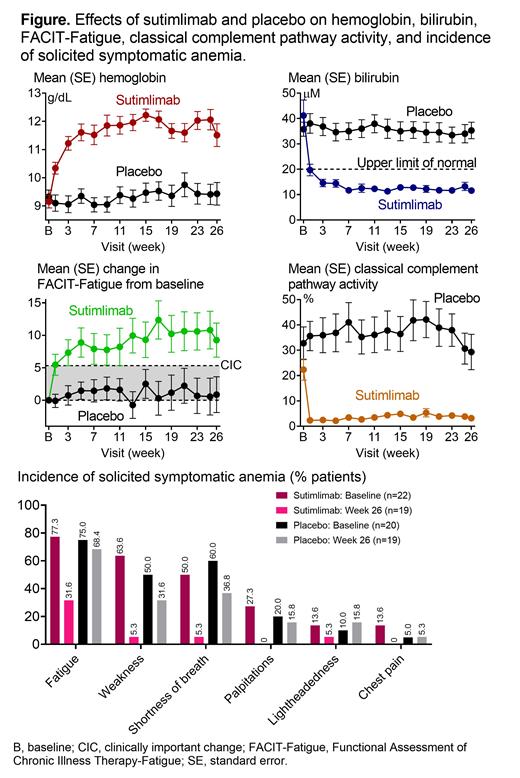
Results: A total of 16 (73%) sutimlimab patients met the composite primary endpoint criteria, compared with 3 (15%) PBO patients (odds ratio, 15.9 [95% confidence interval, 2.9-88.0; P<0.001]). At TAT, 16 (73%) sutimlimab patients had Hb ≥2 g/dL from BL, compared with 2 (10%) PBO patients. One (5%) patient in the sutimlimab arm and 4 (20%) patients in the PBO arm received transfusions from Weeks 5 to 26. Sutimlimab, but not PBO, increased mean Hb and FACIT-Fatigue, and normalized mean bilirubin by Week 1, which were sustained to TAT (Figure). At TAT, the least squares mean (standard error) difference in Hb and FACIT-Fatigue between sutimlimab and PBO was 2.6 (0.4) g/dL (P<0.001) and 8.9 (2.5) points (P<0.001). Sutimlimab led to improvements in additional hemolysis markers, decreased lactate dehydrogenase levels and reticulocyte counts, and increased haptoglobin levels, from BL to TAT (not seen with PBO). For the sutimlimab patients, incidence of solicited symptomatic anemia symptoms reduced from BL to Week 26 for all components; incidence of weakness and shortness of breath reduced in the PBO arm (Figure). In the sutimlimab arm, improvements in anemia, hemolysis, and fatigue coincided with normalized C4 levels and near-complete classical pathway inhibition, with reductions in classical pathway activity (Wieslab) and CH50 levels. C1q levels remained unchanged from BL to Week 26, suggesting pro-phagocytic functions of C1q were unaffected by sutimlimab. PBO arm PD outcomes were unaffected. Twenty-one (96%) sutimlimab patients and 20 (100%) PBO patients experienced ≥1 treatment-emergent adverse event (TEAE). Three sutimlimab (14%) and 1 PBO (5%) patient had ≥1 serious TEAE (TESAE); 1 TESAE of cerebral venous thrombosis assessed as sutimlimab-related by the investigator. No deaths were reported. Serious infections, but no meningococcal infections, were reported. No TESAE of hypersensitivity or anaphylaxis, or TEAE suggestive of the development/worsening of autoimmune disease, or systemic lupus erythematosus, were reported. Three sutimlimab patients (none in PBO) discontinued from study Part A due to TEAE: acrocyanosis and Raynaud's phenomenon (n=1), increased blood IgM (n=1), and infusion-related reaction (n=1). TEAEs reported more frequently in the sutimlimab arm than in PBO with a difference of ≥3 patients between groups were hypertension, headache, Raynaud's phenomenon, rhinitis, and acrocyanosis. Two sutimlimab patients developed transient, low-titer, treatment-emergent anti-drug antibodies, which did not correlate with pharmacokinetics or clinical response, suggesting the immunogenicity risk of sutimlimab is low.
Conclusion: Sutimlimab, but not placebo, rapidly halted hemolysis, markedly increased Hb, and improved QOL. Sutimlimab was generally well tolerated. Results from CADENZA, the first PBO-controlled trial in CAD, alongside the single-arm CARDINAL study, support targeting C1s in CAD.
Roeth: Apellis Pharmaceuticals: Consultancy, Honoraria; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Bioverativ, a Sanofi company: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Kira: Consultancy, Honoraria. Berentsen: Sanofi: Consultancy, Honoraria; Bioverativ: Consultancy, Honoraria; Apellis Pharmaceuticals: Consultancy, Honoraria; Alexion Pharmaceuticals, Inc: Honoraria; Janssen-Cilag: Honoraria; True North Therapeutics: Consultancy; Mundipharma: Research Funding. Barcellini: Alexion Pharmaceuticals, Inc: Membership on an entity's Board of Directors or advisory committees, Other: Invited speaker, Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Invited speaker, Research Funding; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. D'Sa: Janssen: Honoraria, Other: Speaker fees, Research Funding; BeiGene: Honoraria, Other: Speaker fees, Research Funding; Sanofi: Honoraria, Other: Speaker fees, Research Funding. Jilma: True North Therapeutics: Consultancy, Other: Reimbursement for travel costs for scientific presentations; Bioverativ: Consultancy, Other: Reimbursement for travel costs for scientific presentations; Sanofi: Consultancy, Other: Reimbursement for travel costs for scientific presentations. Michel: Alexion Pharmaceuticals, Inc: Consultancy; Rigel: Consultancy; Bioverativ: Consultancy. Weitz: Apellis: Consultancy; Sanofi: Consultancy; Alexion: Consultancy, Speakers Bureau. Nishimura: Biocryst: Consultancy; Apellis: Consultancy; Novartis: Consultancy; Chugai: Consultancy; Roche: Consultancy; Alexion: Consultancy; Sanofi: Consultancy. Vos: Celgene: Other: Travel reimbursement; Sanofi: Membership on an entity's Board of Directors or advisory committees. Storek: Sanofi: Current Employment, Other: May hold shares and/or stock options with Sanofi. Wong: Sanofi: Current Employment, Other: May hold shares and/or stock options with Sanofi. Patel: Sanofi: Ended employment in the past 24 months, Other: May hold shares and/or stock options with Sanofi. Schaible: Sanofi: Ended employment in the past 24 months, Other: May hold shares and/or stock options with Sanofi. Jiang: Sanofi: Current Employment, Other: May hold shares and/or stock options with Sanofi. Vagge: IQVIA: Current Employment; Sanofi: Other: Provided safety analysis and support under contract. Wardecki: Sanofi: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: May hold shares and/or stock options with Sanofi. Shafer: Sanofi: Current Employment, Other: May hold shares and/or stock options with Sanofi. Lee: Sanofi: Current Employment, Other: May hold shares and/or stock options with Sanofi. Broome: Rigel: Honoraria, Research Funding; Cellphire: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bioverativ: Honoraria, Research Funding; Alexion Pharmaceuticals Inc.: Honoraria, Research Funding; Sanofi Genzyme: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal